binimetinib
Appearance
English
[edit]Etymology
[edit]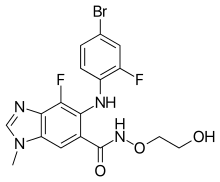
From bini- (a unique prefix) + -metinib (suffix indicating an inhibitor of mitogen-activated protein kinase kinase (MEK)).
Pronunciation
[edit]- (Received Pronunciation) IPA(key): /bɪnɪˈmɛtɪnɪb/
- (General American) IPA(key): /bɪnɪˈmɛtɪnɪb/, [-ɾə-]
Audio (General American): (file) - Hyphenation: bi‧ni‧me‧ti‧nib
Noun
[edit]binimetinib (uncountable)
- (pharmacology) A drug with the molecular formula C₁₇H₁₅BrF₂N₄O₃ that inhibits mitogen-activated protein kinase kinase (MEK), and is approved for use in combination with encorafenib to treat certain melanomas. [from early 21st c.]
- 2017 February 28, Johanna C. Bendell et al., “A Phase 1 Dose-escalation and Expansion Study of Binimetinib (MEK162), a Potent and Selective Oral MEK1/2 Inhibitor”, in British Journal of Cancer, volume 116, Edinburgh: Nature Research, , →ISSN, →OCLC, page 576, column 1:
- Binimetinib (MEK162; ARRY-438162) is a potent, adenosine triphosphate-uncompetitive, highly selective allosteric inhibitor of MEK1/2 with demonstrated on-target activity in vitro and in vivo, including models of cancer [...] In vivo, binimetinib displays broad anti-tumour activity in xenograft models derived from melanoma, colorectal cancer, non-small cell lung cancer (NSCLC), fibrosarcoma, cholangiocarcinoma, and pancreatic cancer. These non-clinical data support the use of binimetinib in a wide variety of tumour types, with a priority in tumours with aberrantly activated MAPK pathway signalling.
- 2017 April 1, Reinhard Dummer et al., “Binimetinib versus Dacarbazine in Patients with Advanced NRAS-mutant Melanoma (NEMO): A Multicentre, Open-label, Randomised, Phase 3 Trial”, in David Collingridge, editor, The Lancet Oncology, volume 18, number 4, London: Lancet Publishing Group, , →ISSN, →OCLC, pages 435–445:
- Binimetinib improved progression-free survival compared with dacarbazine and was tolerable. Binimetinib might represent a new treatment option for patients with NRAS-mutant melanoma after failure of immunotherapy.
- 2018, Laurence Brunton, Bjorn Knollman, Randa Hilal-Dandan, Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 13th edition, New York, N.Y.: McGraw Hill Professional, →ISBN, page 1212:
- Under development are binimetinib, a MEK inhibitor being studied in patients with NRAS-mutant melanoma, and encorafinib, a BRAF inhibitor being used in combination with binimetinib for patients with BRAF-mutant melanoma.
- 2018, Shane Y. Morita et al., Textbook of General Surgical Oncology, New York, N.Y.: McGraw Hill Professional, →ISBN, page 187:
- The initial report of the phase I/II combination trial of encorafenib and binimetinib reported on 9 BRAF inhibitor naive and 14 BRAF inhibitor pretreated patients with metastatic BRAF mutant melanoma across a range of doses.
- 2019 July 20, Heidi Finnes, “Binimetinib (Mektovi®)”, in Oncology Times: The Newspaper for Specialists in Cancer, volume 41, number 14, Philadelphia, Pa.: Lippincott Williams & Wilkins, , →ISSN, →OCLC, page 8:
- Binimetinib is an oral reversible inhibitor of mitogen-activated extracellular signal-regulated kinase (MEK) 1/2. [...] Binimetinib inhibits MEK 1/2 downstream from BRAF and halts cancer cell signaling and survival. [...] Binimetinib is approved in combination with encorafenib, a BRAF inhibitor, for the treatment of unresectable or metastatic melanoma with a BRAF V600E/K mutation, as detected by an FDA-approved test.
- 2020, Edward Chu, Vincent T[heodore] DeVita, Jr., “Chemotherapeutic and Biologic Drugs”, in Physicians’ Cancer Chemotherapy Drug Manual 2021, Burlington, Mass.: Jones & Bartlett Learning, →ISBN, →ISSN, page 77:
- Special Considerations [...] 7. Monitor patients for an increased risk of new primary cancers, both cutaneous and non-cutaneous, while on therapy and for up to 6 months following the last dose of binimetinib. [...] 10. Baseline and periodic CPK levels while on therapy, as rhabdomyolysis has been observed with binimetinib therapy.
- 2020, Gail M. Wilkes, Margaret Barton-Burke, “Molecular Targeted Therapy”, in Oncology Nursing Drug Handbook 2020–2021, Burlington, Mass.: Jones & Bartlett Learning, →ISBN, →ISSN, page 548:
- Binimetinib is given in combination with encorafenib as each drug targets a different kinase in the RAS/RAF/MEK/ERK pathway to stop malignant cell proliferation and to tell the cells to die (undergo apoptosis). [...] Terminal half-life of binimetinib is 3.5 hours. [...] No clinically significant changes in binimetinib exposure were seen in patients with severe renal impairment.
Translations
[edit]drug that inhibits mitogen-activated protein kinase kinase
|
Further reading
[edit] binimetinib on Wikipedia.Wikipedia
binimetinib on Wikipedia.Wikipedia
